Immunotherapy has led to substantial advances in cancer therapy in recent years. Still, unpredictable response rates and immune-related adverse events have hampered the widespread use of immune checkpoint therapy to treat cancers. To tackle these challenges, sponsors are increasingly looking to combination therapies as a strategy for improving response and overcoming resistance.
In our previous post, we addressed the goals of immunotherapy, characteristics of an ideal target, and major approaches in use today. In this blog, we examine the rationale for combination therapies, mechanisms of resistance, and where we go from here.
The rationale for combination therapies
The interactions between cancer and the immune system are complex and involve a series of stepwise events referred to as the cancer-immunity cycle.[1] The multitude of both stimulatory and inhibitory factors involved in the cycle offers a wide range of potential therapeutic targets, some examples of which are highlighted in Figure 1.1
Figure 1. Intervening in the cancer-immunity cycle1
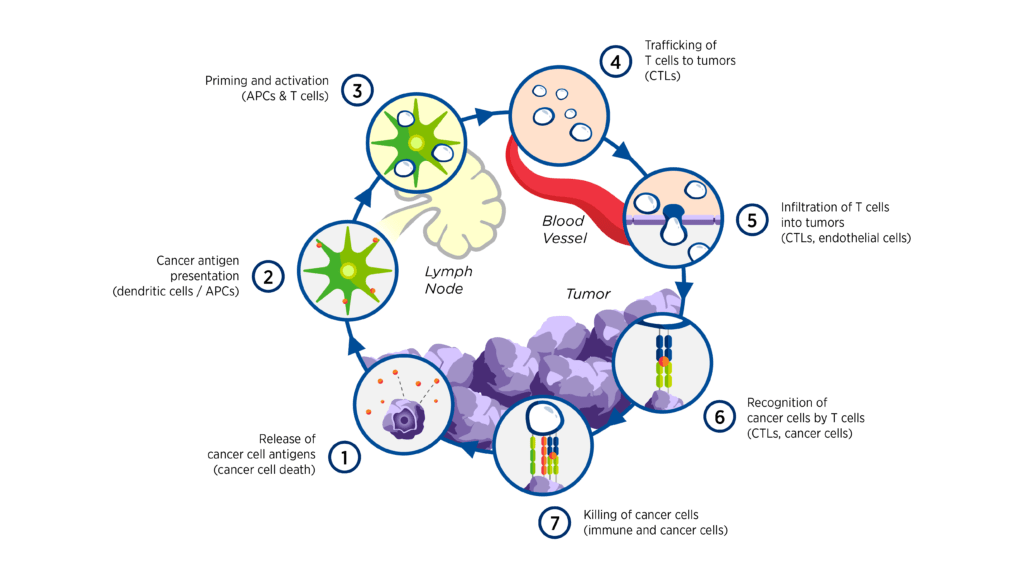
The complexity of the immune response to cancer also provides a strong rationale for combination therapies. Examples of combination treatments may include:
- Immunotherapy/immunotherapy. Two immunotherapies targeting different immune checkpoints
- Immunotherapy/chemotherapy. Direct killing of tumor cells with chemotherapy may help activate the immune system, potentially leading to an additive effect for immunotherapy
- Immunotherapy/targeted therapy. For example, anti-VEGF therapy may stimulate the immune system and inhibit tumor vascularization, creating a possible synergistic effect with immunotherapy
Exploring how biomarkers interact may aid in the design of combination strategies. Various clinical trials are studying the sequential treatment of immunotherapies before or following chemotherapies to determine if this strategy can turn “cold” non-immunogenic tumors into “hot” tumors, which would then respond to immunotherapy treatment.[2],[3] All these combination therapies aim to modulate the immune-suppressive microenvironment and initiate tumor cell death, recruiting effector T cells to the tumor and thereby increasing the efficacy of the immunotherapy.
Mechanisms of resistance
Resistance to immunotherapy may be primary (failure to respond) or secondary/acquired (relapse after successful treatment). Approaches for optimizing response and minimizing resistance to cancer immunotherapies include developing biomarkers to assist with patient selection, altering the tumor microenvironment, and educating healthcare practitioners to check for a delayed response using iRECIST criteria.
Another mechanism of resistance to immunotherapy is adaptive resistance, which is the escape phenomenon whereby tumors evade T-cell recognition. This phenomenon can occur due to tumor secretion of immunosuppressive cytokines or immune system exhaustion, in which tumor growth exceeds the immune system’s ability to keep up or when immune checkpoints are upregulated.
It is important to remember that the immune response is dynamic and constantly evolving in each patient, either due to the patient’s own environmental and genetic factors or as a result of treatment interventions. This complex, dynamic relationship between cancer and the immune system has given rise to the age of immuno-oncology and a new era of cancer treatment that brings unique challenges and opportunities. Anti-tumor immune responses that are ongoing throughout the course of a patient’s disease may be affected by many factors, and the establishment of resistance mechanisms relevant to immunotherapeutic failure may predate immunotherapy challenge.
Future of immunotherapy
Last year capped an incredible decade for cancer immunotherapy, and despite the obstacles, the future for these treatments is a bright one. Immunotherapy holds the promise of transforming cancer into a more manageable condition with a better prognosis for patients. New research is placing increasing importance on developing better lab models to study the immune response and the tumor microenvironment. Additionally, technological advances like liquid biopsies and novel, non-invasive imaging strategies also have the potential to improve our ability to discover and validate new biomarkers, making it easier for doctors to incorporate them into standard clinical practice.[4]
Premier Research has completed more than 170 hematology and oncology clinical trials through every phase over the past five years, with more than 40 percent involving an immunotherapy component. To learn more about our oncology expertise, click here.
[1] Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39(1):1-10.
[2] Phase 2 study for previously untreated subjects with non-small-cell lung cancer (NSCLC) or small-cell lung cancer. ClinicalTrials.gov, U.S. National Library of Medicine, National Institutes of Health (NIH); 2018. ClinicalTrials.gov Identifier NCT00527735.
[3] Nivolumab after induction treatment in triple-negative breast cancer (TNBC) patients (TONIC). ClinicalTrials.gov, U.S. National Library of Medicine, National Institutes of Health (NIH); 2021. ClinicalTrials.gov Identifier NCT02499367.
[4] Wolchok JD, et al. The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 2020;30(2):507-519.
