Chimeric antigen receptor (CAR) therapies use CAR T cells, a patient’s own immune cells that are programmed to recognize and kill cancer cells throughout the body. Beginning in 2017 with the approvals of tisagenlecleucel (Kymriah™) and axicabtagene ciloleucel (Yescarta™), CAR T-cell therapies have changed the treatment paradigm for patients with certain hematologic malignancies.
Since those initial approvals, three other CAR T-cell therapies have been approved for various hematologic malignancies: brexucabtagene autoleucel (Tecartus™), lisocabtagene maraleucel (Breyanzi®), and most recently, idecabtagene vicleucel (Abecma®). Despite the remarkable clinical responses seen in patients with hematological malignancies, many challenges limit the therapeutic efficacy of current CAR T-cell therapies and their use in solid tumors.
What is CAR T-cell therapy?
CAR T-cell therapy involves re-engineering a patient’s own T cells to recognize and eradicate cancer. These T cells are genetically altered to express artificial receptors that enable the T cells to bind to a specific antigen on the patient’s tumor cells and kill them. Unlike T-cell receptor-mediated immune reactions, CAR T-cell-mediated immune reactions lead to direct recognition of extracellular tumor-associated antigens; however, immunogenicity can be challenging.
Figure 1. Normal vs. CAR T cell[1],[2]
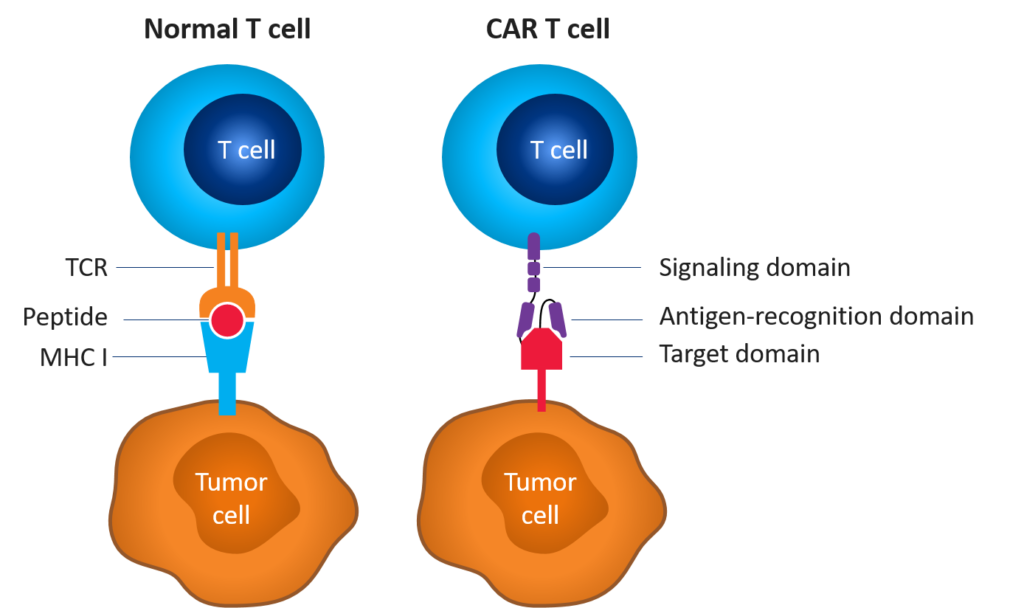
Development of CAR T-cell therapies
To date, five CAR T-cell therapies have been approved by the FDA:
- Tisagenlecleucel, marketed as Kymriah™, is approved for the treatment of adults with relapsed or refractory diffuse large B-cell lymphoma and young adult patients up to age 25 with relapsed or refractory acute lymphoblastic leukemia
- Axicabtagene ciloleucel, known by the brand name Yescarta™, is approved for the treatment of adults with certain types of B-cell lymphoma who have either not responded to or have relapsed following two or more lines of systemic therapy
- Brexucabtagene autoleucel, marketed under the brand name Tecartus™, is approved to treat patients with mantle cell lymphoma
- Lisocabtagene maraleucel, known by the brand name Breyanzi®, is approved to treat adult patients with relapsed or refractory large B-cell lymphoma
- Idecabtagene vicleucel, also known as Abecma®, is approved to treat patients with multiple myeloma
Clinical development of CAR T-cell therapies has accelerated significantly over the past decade, with 229 open or planned trials in the U.S. alone and over 950 trials globally as of June 2021.[3]
Figure 2. CAR T-cell trials by region as of June 20213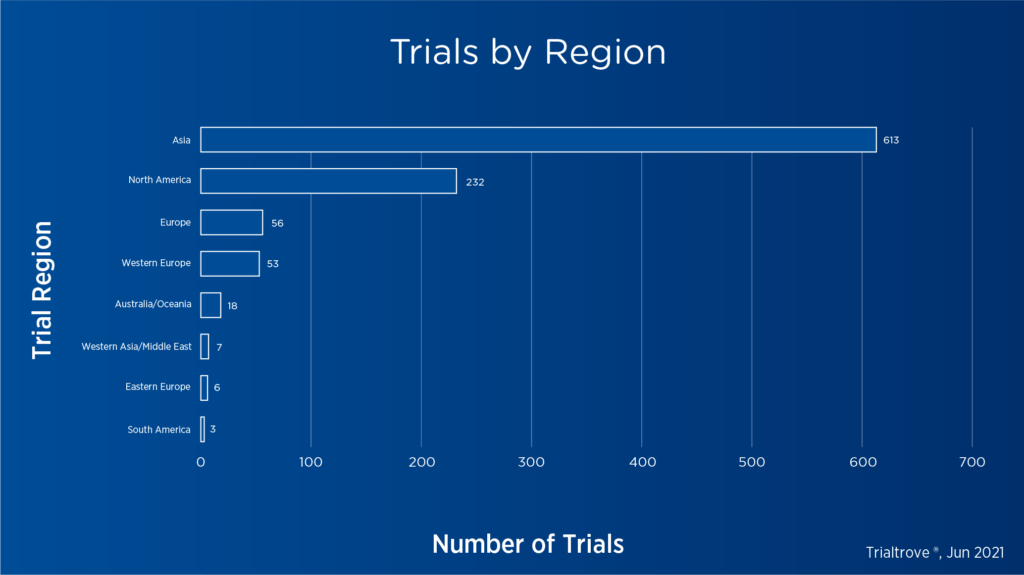
Click image to expand
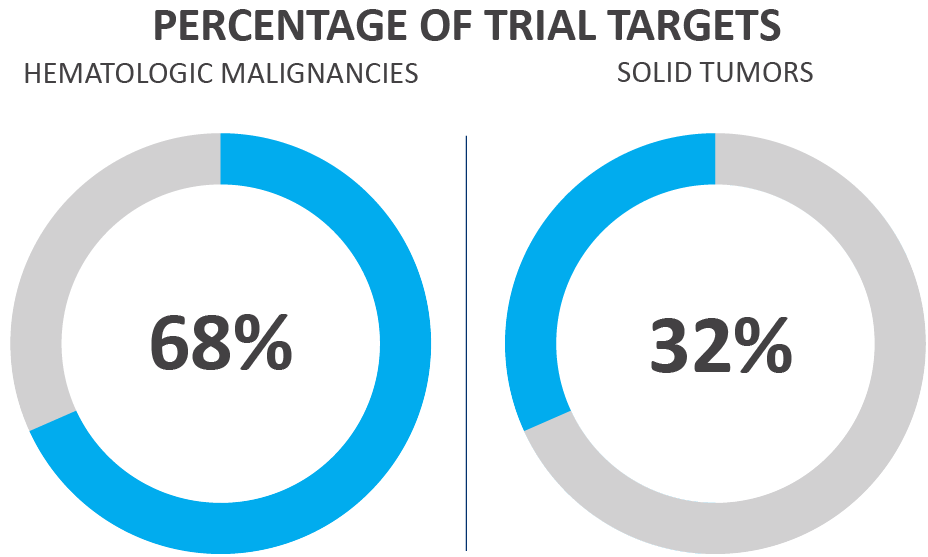
Figure 3. Oncological CAR T-cell trials by target type as of June 20213
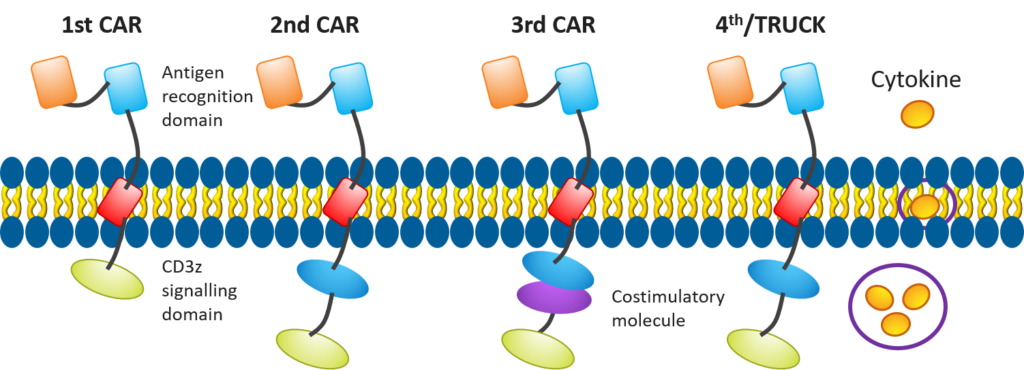
 In recent years, researchers have been making efforts to enhance the efficacy of CAR T cell-based therapies, such as improving the structures of CAR T cells (see Figure 4) to include additional costimulatory molecules, chemokines, or developing mechanisms — like suicide switches — to make these treatments more effective and safer.
In recent years, researchers have been making efforts to enhance the efficacy of CAR T cell-based therapies, such as improving the structures of CAR T cells (see Figure 4) to include additional costimulatory molecules, chemokines, or developing mechanisms — like suicide switches — to make these treatments more effective and safer.
Figure 4. Evolution in CAR T-cell design[4]
CAR T-cell therapies offer many advantages but also require unique considerations, including having an effective strategy in place for managing adverse events such as cytokine release syndrome (CRS). We cover this and more in Part 2 of this blog series.
With more than 170 hematology and oncology clinical trials completed through every phase in the past five years, including 19 oncological cell and gene projects, Premier Research’s clinical trial expertise helps you get from brilliant idea to life-changing reality. Click here to learn more.
[1] Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Biotechnol 2013;31:999-1008.
[2] Adapted from Locke FL, Gardner R, Neelapu SS. Test driving CARs: optimizing outcomes. Medscape. 2017 December 20. Retrieved from https://www.medscape.org/viewarticle/890215_transcript
[3] Trialtrove: gold standard clinical trials intelligence. Informa PLC 2021.
[4] Adapted from Xu D, Jin G, Chai D, et al. The development of CAR design for tumor CAR-T cell therapy. Oncotarget 2018;9(17):13991-14004.
