While clinical investigations of medical devices and investigational drugs have their differences, what they do have in common is the goal of safeguarding the rights and welfare of study participants while bringing safe and effective products to market as quickly and efficiently as possible.
Not all medical devices require a clinical trial, depending on their risk stratification. But when a clinical trial is required, device sponsors will need to follow many of the same regulatory requirements as for pharmaceutical trials, including:
- 21 CFR Part 11 – Electronic Records; Electronic Signatures, which sets the criteria under which the FDA considers electronic records, electronic signatures and handwritten signatures executed to electronic records to be trustworthy, reliable, and generally equivalent to paper records and handwritten signatures executed on paper
- 21 CFR Part 50 – Protection of Human Subjects, which covers informed consent and additional safeguards for children in clinical investigations
- 21 CFR Part 54 – Financial Disclosure by Clinical Investigators, which requires applicants to disclose certain financial arrangements between sponsors and clinical investigators as well as certain interests of the clinical investigators in the product under study or in the sponsor
- 21 CFR Part 56 – Institutional Review Boards, which contains the general standards for the composition, operation, and responsibility of an Institutional Review Board that reviews clinical investigations supporting applications for research or marketing permits for products regulated by the FDA, including drugs and medical devices for human use
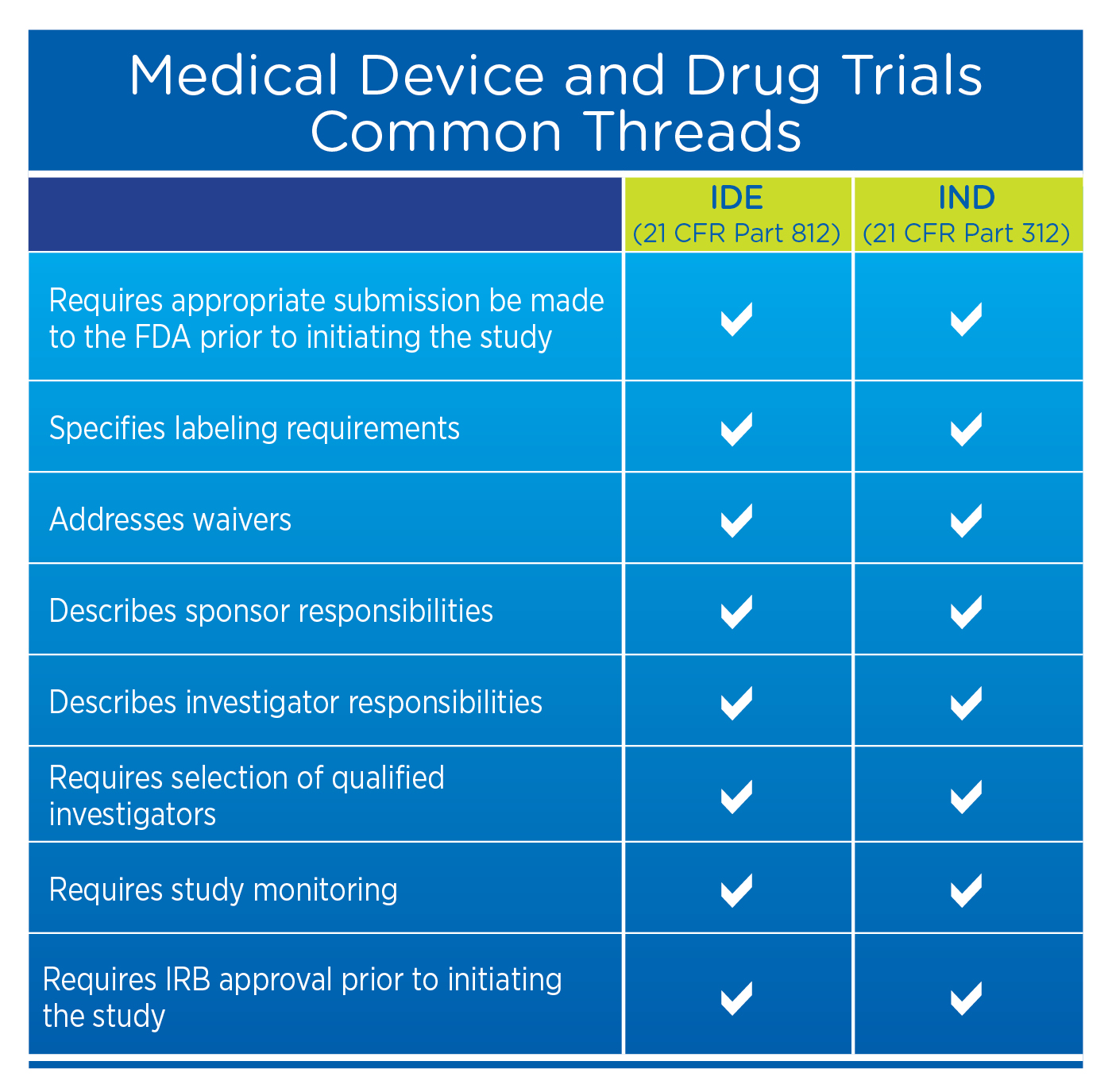
While device sponsors will not need to complete an Investigational New Drug Application (IND, as per 21 CFR Part 312), they will need to complete an Investigational Device Exemption (IDE, as per 21 CFR Part 812) which has many similar requirements, as seen in the table below.
How the Federal Regulatory Requirements for Devices and Drugs are Similar
However, there are key differences between medical device and drug trials, and device companies seeking to outsource a clinical trial to a contract research organization (CRO) should be sure that the CRO has relevant experience and suitably qualified staff to handle all the requirements.
