PremierOne Ecosystem
A holistic technology ecosystem fuels biotech innovation.
Delivering clarity amid the complexity
The PremierOne Ecosystem aggregates all the data captured within our ePremier environment. The ecosystem also represents Premier Research’s processes for data analysis related to administrative and financial data, study management data, and patient data.
Fully integrated data support analysis across study functions
- Captures administrative and financial data (contracts, resourcing, vendors, and invoices); study management data (timelines, CRMs, CTMS, eISF, and TMF); and patient data (EDC, ePRO, laboratory, IVRS/IWRS, eCOA, and mHealth devices)
- System-agnostic CTMS+ streamlines study workflow, including visit scheduling, report generation, and action items
- Generates audit trail and eTMF-ready documentation with protocol deviation tracking and follow-up
- Enables risk management through aggregated site and patient data
ePremier makes data capture logical, obvious, and easy
- Captures the right data from the specified system at the right time
- Aggregates data across all sites to provide simultaneous access across study teams
- Verifies the data as complete, accurate, and specific to your study outcomes
- Supports critical thinking with study-, country-, and site-specific data flows
- Enables consistent, centralized routine assessment of scientific and procedural parameters
- Improves overall data quality by identifying outliers and trends in near real-time
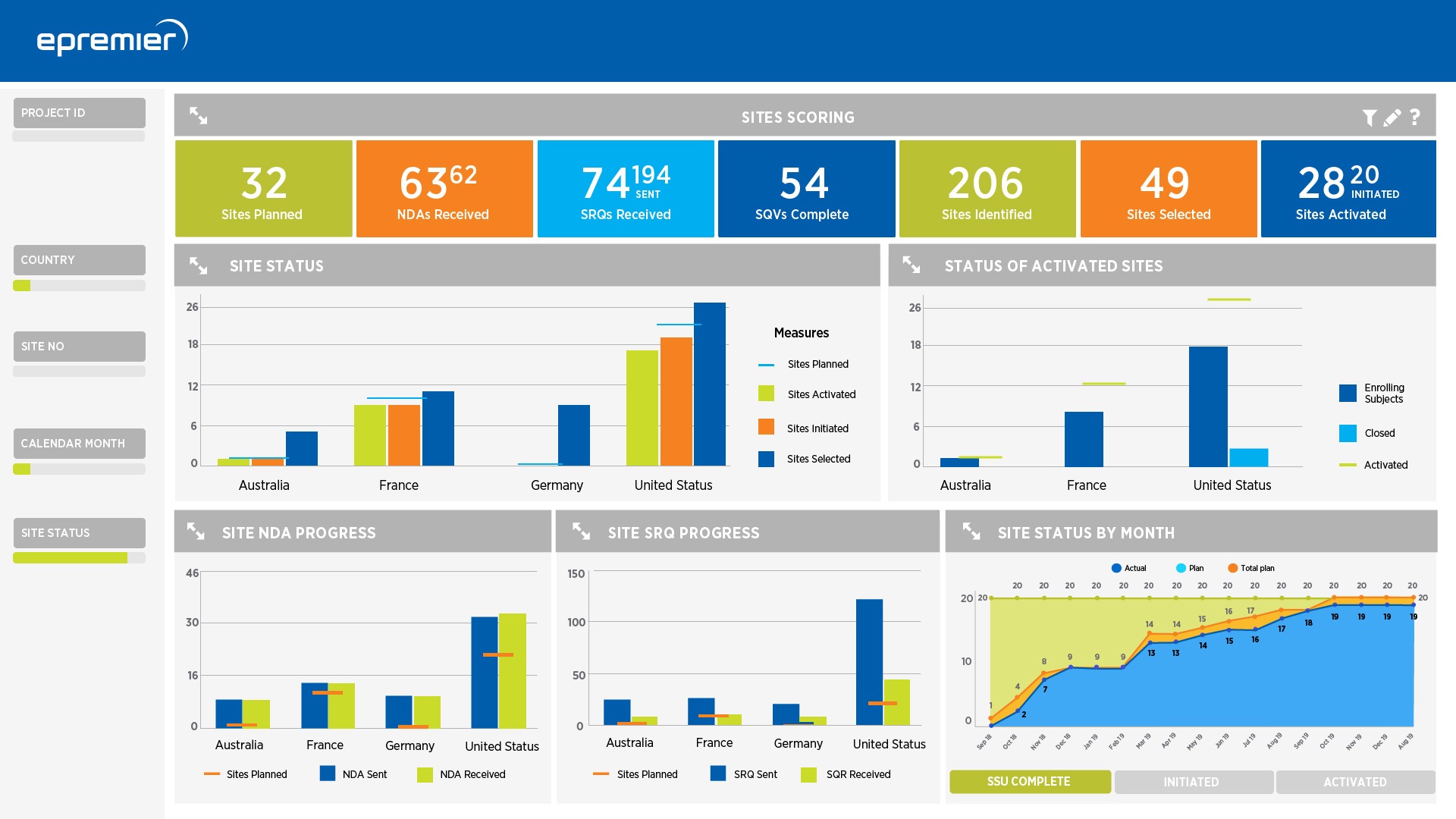
Highly trained clinical data scientists and in-process analytics inform study decisions in real-time
- Customized dashboards visualize insights from multiple sources summarized for transparency and ease
- In-depth evaluation of KRIs and other data review disclose the root cause of issues
- Clear communications address systemic process issues and site behaviors
Clinical trial monitoring needs have shifted
Today’s clinical studies may have multiple arms, broadly dispersed enrolled patients, or patients who are unable or unwilling to visit sites. When teams don’t agree on process, they work in silos — making it impossible to predict and assess risk and often resulting in trial delays from poor or untimely communication. The PremierOne Ecosystem was designed specifically to eliminate these issues.
The PremierOne Ecosystem offers a collaborative monitoring process, which combines central and on-site monitoring to optimize data quality management and address regulatory requirements. Our highly trained clinical data scientists monitor data and analytics across sites and patients to identify specific concerns and ensure patient records and study documentation are current, accurate, and high quality.
Brochure
PremierOne Ecosystem
Both flexible and precise, the PremierOne Ecosystem enables processes, applies rigorous standards, and supports the careful management of all study data to minimize risk and meet regulatory requirements. Learn more in our brochure.
Explainer Video
ePremier In Action
We understand how important it is to capture the right data at the right time, capture the data necessary to solve a problem, and ensure that data is complete, accurate, and conclusive. That’s why we developed the ePremier Integration Hub.
Technology-Enabled Clinical R&D Services:
Related Capabilities
Adding value at every step in the process, we provide expertise in strategic product development, global regulatory consulting, and clinical R&D to ensure your trial’s data integrity, process efficiency, and timely analytics and reporting.
Strategic Product Development

Global Regulatory Consulting

Clinical Research & Development
Connect with us
Ready to make your vision a reality? So are we. Drop us a line to learn more about how we can help.