Despite advances in our understanding of the complex genetic, molecular, and immunological factors which lead to cancer, the success and likelihood of approval rates for oncology remain low. According to a recent study performed by the Biotechnology Innovation Organization (BIO), nearly one-third of drugs entering phase 2 studies between 2006 and 2015 failed to progress. Of the 14 major disease areas covered by the study, oncology drugs had the lowest likelihood of approval at just 5.1 percent but were also the fastest to get approved. Within oncology, treatments for hematological cancers were twice as likely to be approved as therapies for solid tumors.1
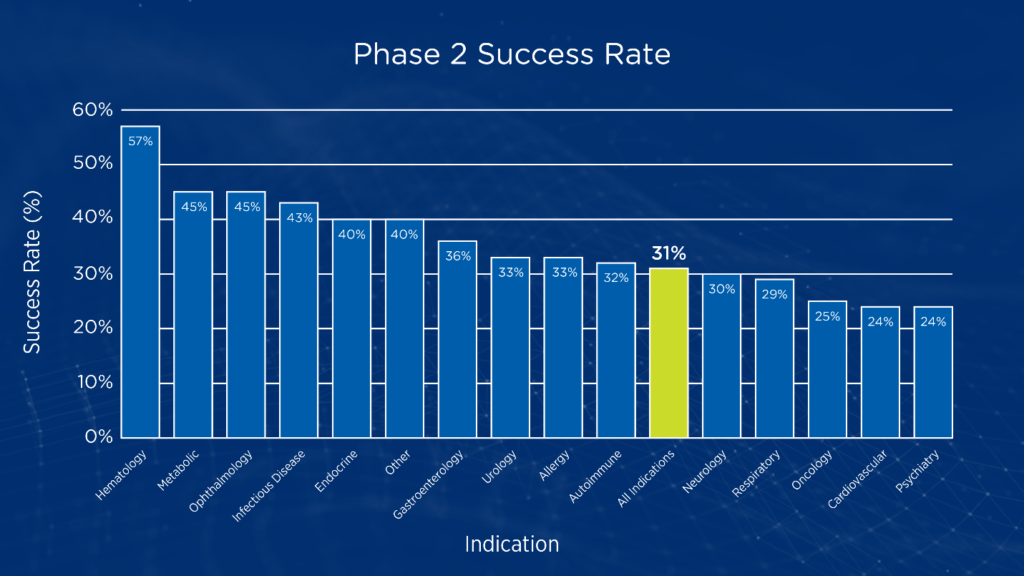
While phase 1 studies typically focus on dose-finding and establishment of an initial safety profile, phase 2 studies are often the first stage of clinical development aimed at testing proof-of-concept in human subjects. Phase 2 studies are also the point where sponsors must decide whether to pursue large, costly phase 3 studies or terminate development. In oncology, the likelihood of success when transitioning from phase 2 to phase 3 studies is only 24.6 percent (see Figure 1).1
Figure 1. Phase 2 transition success rates by disease area1
Why early oncology drugs fail
Many factors contribute to the low rate of phase 2 transition success in oncology trials, including inaccurate maximum tolerated dose (MTD), insufficient data to provide statistical confidence that the drug will be effective in the selected indication, and failure to explore combinations that include standard care. In today’s oncology landscape with its increasing emphasis on precision medicine, the lack of a validated biomarker for pre-screening study participants to identify those most likely to respond can also adversely impact the success of phase 2 studies. According to the BIO study, the use of selection biomarkers increases the probability of phase 2 success from 28 percent to 46 percent across disease areas.1
How to optimize the likelihood of success
With the emergence of precision medicine, there has been a shift in how early oncology trials are conducted, including an increasing number of phase 1 trials reporting preliminary response rates. This change is due, at least in part, to adaptive trial designs, which aim to limit the number of patients exposed to ineffective doses or treatments while enabling early detection of potential efficacy signals.
Advances in our understanding of the genetic underpinnings of cancer have led to a growing number of phase 1 studies that are driven by a common mechanism of action or molecular alterations rather than specific disease type. Consequently, in addition to the usual objectives of dose-finding and safety assessment, these phase 1 studies also aim to select the most promising indication(s) to be pursued in phase 2 studies. Adaptive design approaches are well suited for helping sponsors optimize dose and dosing regimen, while also narrowing down the indications of interest.
Historically, most phase 1 oncology trials relied on standard 3+3 dose escalation designs for determining a recommended phase 2 dose (R2PD). Unfortunately, research suggests that as few as one-third of trials using the 3+3 design actually succeeded in identifying the MTD. Moreover, this dose-escalation method comes with the risk of treating a higher than expected percentage of patients at subtherapeutic doses.2 Another potential limitation of standard 3+3 dose escalation designs is that targeted therapies and immunotherapies may not produce a dose-limiting toxicity and may require an endpoint of optimal biological dose (OBD) rather than MTD.
An adaptive design alternative to the standard 3+3 dose escalation design is the continual reassessment method, which uses statistical model-based dose-escalation algorithms to help estimate the MTD. With this method, progressive algorithms allow a change in dose level after each patient is treated. As dose escalations may occur more quickly, it may be possible to determine an MTD or R2PD more quickly and more accurately than with a fixed-sample design.3
Learn more about adaptive design approaches in early oncology trials
The continuous reassessment method is just one example of an adaptive design that may help sponsors optimize early oncology studies. To learn more about how adaptive designs can be used to accelerate the timeline to detection of efficacy signals, download our white paper Adaptive Trial Designs in Early Oncology: Minimizing Risk & Accelerating Timelines.
[1] Thomas DW, et al. Clinical development success rates 2006-2015. Biotechnology Innovation Organization, Washington DC. June 2016. Accessed May 16, 2020.
[2] Reiner E, Paoletti X, O’Quigley J. Operating characteristics of the standard phase I clinical trial design. Comput Stat Data Anal. 1999;30(3):303-315.
[3] Wheeler GM, et al. How to design a dose-finding study using the continual reassessment method. BMC Med Res Methodol. 2019;19:18.
