Dermatology clinical research is undergoing a rapid transformation in response to new demands for clinical trials that are more justifiable, safer, and less burdensome for patients. In dermatology, a patient-focused approach can be applied to all phases of the research process, from the earliest stages of discovery and pre-clinical testing to clinical trials, regulatory approval, and post-marketing studies. Each of these steps can benefit significantly from patient input that informs more relevant and feasible research activities.
In this blog post, we explore a strategic, patient-focused approach to planning and implementing dermatology clinical trials.
Understanding the importance of a patient focus
At its core, a patient-focused approach involves designing a clinical trial around patient characteristics and needs. In addition to being the right thing to do, actively involving the patient in the research and development process is also necessary for meeting the expectations of regulators, providers, and payers. In June 2020, the FDA released guidance on collecting comprehensive and representative patient experience data stressing the importance of reducing patient burden in trials, beginning with designing a well-vetted trial that incorporates patient input. This document is the first in a series of four patient-focused drug development guidance documents to address how stakeholders should collect and submit patient experience data and other relevant patient and caregiver input to support both product development and regulatory decision making.1
The importance of patient feedback also extends beyond product development and regulatory approval. With the trend toward personalized medicine, providers rely not only on available clinical trial data but also on their own clinical experience with individual patients to formulate their opinions on therapeutic options. Moreover, payer decisions in the dermatology space are increasingly driven by real-world evidence, where the relationship between patient adherence and subsequent outcomes is a key consideration.
Finding consistency between sponsor and patient priorities
Sponsors may perceive that patient perspectives or priorities related to clinical trials are different from their own, but that is usually not the case.
When patients are considering enrollment in a dermatology clinical trial, they are seeking a correct diagnosis, potential access to a successful treatment, and hope for improvement in their skin condition and their quality of life—all without significant additional burden related to study participation. Patients are also motivated by a philanthropic or altruistic desire to help others with the same condition on a larger, global scale.
For sponsors, clinical trial priorities typically include:
- Good inclusion-exclusion criteria for correct diagnosis and recruitment
- Treatment adherence
- Study retention
- Reliable, high-quality data to support regulatory approval
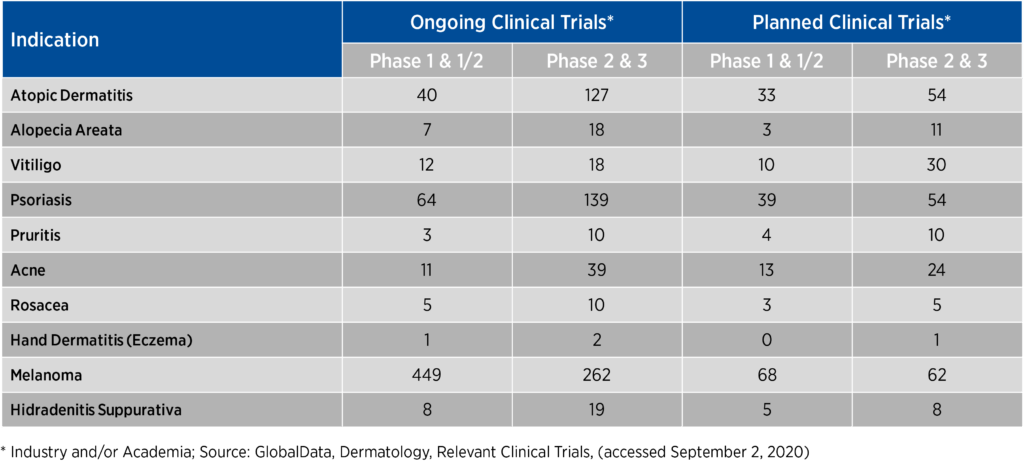
In order to execute on these priorities, sponsors must consider the level of burden associated with study participation and align their perspectives with those of patients. Engaging in direct collaboration with prospective study participants creates not only consistent intentions but also much-needed differentiation in a highly competitive dermatology space where there may be hundreds of trials simultaneously recruiting for certain indications and patient populations (see Figure 1).
Figure 1. Number of ongoing and planned clinical trials in competitive dermatologic indications
Creating a competitive edge by focusing on the patient
To create differentiation and a competitive edge for dermatology clinical trials, sponsors should incorporate a patient-focused approach to study design, recruitment and enrollment, and study implementation.
Study design. Recruiting, enrolling, and retaining the right patient in a dermatology clinical trial begins with creating a patient-focused protocol. Involving patients in a protocol review is an opportunity for sponsors to seek direct feedback from patients on what is feasible and acceptable in terms of trial burden and whether the proposed assessments and endpoints make sense, which is important for the patients’ treatment outcome measures.
Recruitment and enrollment. Patient involvement is also extremely valuable to sponsors when building a strategy for raising trial awareness, creating trial messaging, and the development of all patient-facing documents, from marketing materials to informed consent forms.
Study implementation. A successful study is one that is meaningful to patients and their families and one that is feasible from a participation perspective. To enhance retention, sponsors and contract research organizations must be thoughtful and creative in finding ways to help patients and families manage any potential challenges that accompany participation in the clinical trial. When treated as true partners in the study implementation, patients stay engaged. Some of the activities to consider are regular check-ins with patients, including compliantly keeping patients in the loop about study progress, completion, and study outcomes, regional patient-centric events, and other ways to support them and help to improve their quality-of-life.
In addition, forming strong partnerships with patient organizations and creating patient advisory boards and/or committees can help facilitate these activities. Incorporating technological advancements such as mHealth for virtual visits, e-consent, or wearables and apps for data collection can also help decrease the burden of participation.
Key takeaways
In today’s regulatory environment and competitive clinical trial landscape, incorporating patient input and feedback throughout the dermatology product development process is key. Working with a CRO that has deep dermatology experience and a patient-focused orientation can help sponsors design, develop, and implement studies that align patients’ priorities with the sponsor’s definition of success. To learn more about the convergence of patients, regulations, and study design, view our webinar: Essential Strategies in Dermatology Clinical Trials.
Premier Research has performed more than 110 dermatology studies and worked with thousands of patients over the past five years, we know how to make your study a success. Click here to schedule a consultation with our dermatology experts.
1 U.S. Food and Drug Administration. FDA Patient-Focused Drug Development Guidance Series for Enhancing the Incorporation of the Patient’s Voice in Medical Product Development and Regulatory Decision Making. Available at https://www.fda.gov/drugs/development-approval-process-drugs/fda-patient-focused-drug-development-guidance-series-enhancing-incorporation-patients-voice-medical. Accessed November 18, 2020.
