Immuno-gene therapeutics are transforming the therapeutic landscape of hematological malignancies. The recent approvals of two chimeric antigen receptor (CAR) T-cell therapies—tisagenlecleucel (marketed as Kymriah™) and axicabtagene ciloleucel (marketed as Yescarta™)—mark the beginning of the next revolution in cancer treatment. However, along with demonstrated efficacy in hematologic malignancies, CAR T-cells have the capacity to elicit serious toxicities.
Safety considerations related to CAR T-cells may impact both trial design and trial management, as the adverse events (AEs) associated with immuno-oncology agents differ from those associated with cytotoxic therapies. Consequently, anticipating, preventing and managing toxicity is a key component of clinical studies involving CAR T-cells.
Toxicities Associated with CAR T-Cell Therapies
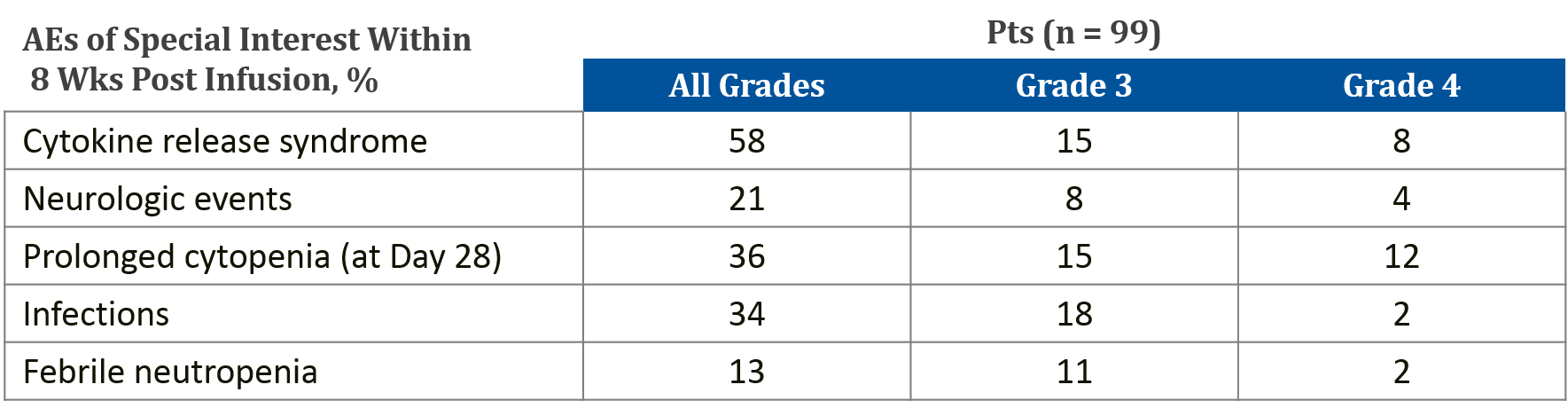
AEs following T-cell-based therapies vary widely and may be immediate, delayed, mild, or severe. In some cases, the toxicities may persist for the lifespan of the genetically modified T-cell.[1] CAR T-cell therapies have clear associations with serious toxicity. To date, cytokine release syndrome (CRS) is the most prevalent AE following CAR T-cell infusion. CRS can range in severity from low-grade constitutional symptoms to life-threatening multi-organ dysfunction. In rare cases, CRS can evolve into hemophagocytic lymphohistiocytosis (HLH) and it has been linked to two reported deaths in clinical trials.[2] The second most common AE is CAR T-cell-related encephalopathy syndrome (CRES). Combination studies with other IO therapies could potentially increase toxicity in terms of both severity and range of AEs.
Designing a CAR T study with Safety in Mind
Regular safety review meetings and Data Safety & Monitoring (DSM) are required for any study involving CAR T, and adjudication of dose-limiting toxicities (DLTs) may be considered.
In addition, the following should be clearly defined in the study protocol:
- Prophylactic measures
- Management and dose adjustments of other medication
- Permissible concomitant medications and supportive care
- Any immediately reportable AEs
- What does not need to be reported, e.g., progression of disease
To overcome safety challenges, it is also important to consider variables in the safety profile, including:
- Concomitant medications
- Disease being studied, prior lines of therapy, and disease burden
- CAR design and production and amplification of the CAR T-cell
- Patient pre-conditioning and administered doses
Toxicity Management with CAR T-Cell Therapy
Advancements in CAR T, such as integration of genetic constructs containing ‘safety (suicide) switches’ or ‘elimination genes’ and ‘remote-controlled’ CARs, may help to limit toxicities. Suicide switches and elimination genes are designed to eliminate target CAR T-cells when life-threatening toxicities develop. Remote-controlled CARs include an inducible gene-regulatory system that enables controlled expression.
Recently, the CAR-T-cell-therapy-associated TOXicity (CARTOX) Working Group developed a set of recommendations for monitoring, grading and managing toxicities in patients treated with CAR T therapy, and these recommendations should inform a patient’s schedule within a protocol design.[4]
These recommendations suggest the following procedures before and during CAR T-cell infusion:4
- Baseline brain MRI to rule out any central nervous system disease
- Central venous access
- Cardiac monitoring by telemetry beginning on the day of CAR T-cell infusion and continuing until CRS resolves
- Tumor lysis precautions for patients with bulky tumors
- Consideration of seizure prophylaxis for therapies known to cause CRES
- Hospitalization for at least seven days after CAR T-cell infusion
Patient monitoring after CAR T-cell infusion should include:4
- Vital signs every four hours, along with close monitoring of oral and intravenous fluid input and urine output and daily measurement of body weight
- Daily review and physical examination
- Daily blood counts, complete metabolic profiling, and coagulation profiling
- Daily C-reactive protein and ferritin levels
- Assessment and grading of CRS twice a day, and whenever the patient’s status changes
- Assessment and grading of CRES using the CAR-TOX 10-point neurological assessment (CARTOX-10) every eight hours
- Maintenance intravenous fluids with normal saline to ensure adequate hydration
*Grade 1 CRS can manifest as fever and/or grade 1 organ toxicity.
‡For grades 2, 3, or 4 CRS, any one of the criteria other than fever is sufficient.
§High-dose vasopressors are defined in Lee DW, et al.5
||Grading of organ toxicities is performed according to Common Terminology Criteria for Adverse Events, version 4.03.
Learn More About the Author
CARTOX-10 involves asking a patient to:4
- Name the year, month, city, hospital and president or prime minister of their home country (5 points)
- Name three nearby objects (3 points)
- Write a standard sentence (1 point)
- Count backwards from 100 by tens (1 point)
Every member of the care team and investigative site staff needs to be trained to recognize the unique toxicities of CAR T-cell therapies and be able to act accordingly. Accurate assessment and prompt, appropriate management of toxicities can help mitigate adverse outcomes, maximizing the benefit of adoptive T-cell therapies while minimizing the risk of life-threatening complications.
Watch our webinar to learn more about planning and operationalizing clinical studies of gene therapy technologies in hemato-oncology.
Citations
[1] Bonifant CL, et al. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016;2:16011.
[2] Hartmann J, et al. Clinical development of CAR T-cells—challenges and opportunities in translating novel innovative treatment concepts. EMBO Mol med 2017;9(9):1183-1197.
[3] Schuster SJ, et al. American Society of Hematology (ASH) Annual Meeting 2017: Abstract 577.
[4] Neelapu SS, et al. Toxicity management after chimeric antigen receptor T cell therapy: one size does not fit all. Nat Rev Clin Oncol 2018 Feb 13. Epub 2018 Feb 13.
[5] Lee DW, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-195.
