What are sponsors in medical device trials responsible for? Pretty much everything. Seriously.
Clinical trials for medical devices involve many complex “moving parts” in the form of different groups of investigators, suppliers, contractors, committees, and other organizations. For a successful trial, sponsors must make sure each works together in harmony.
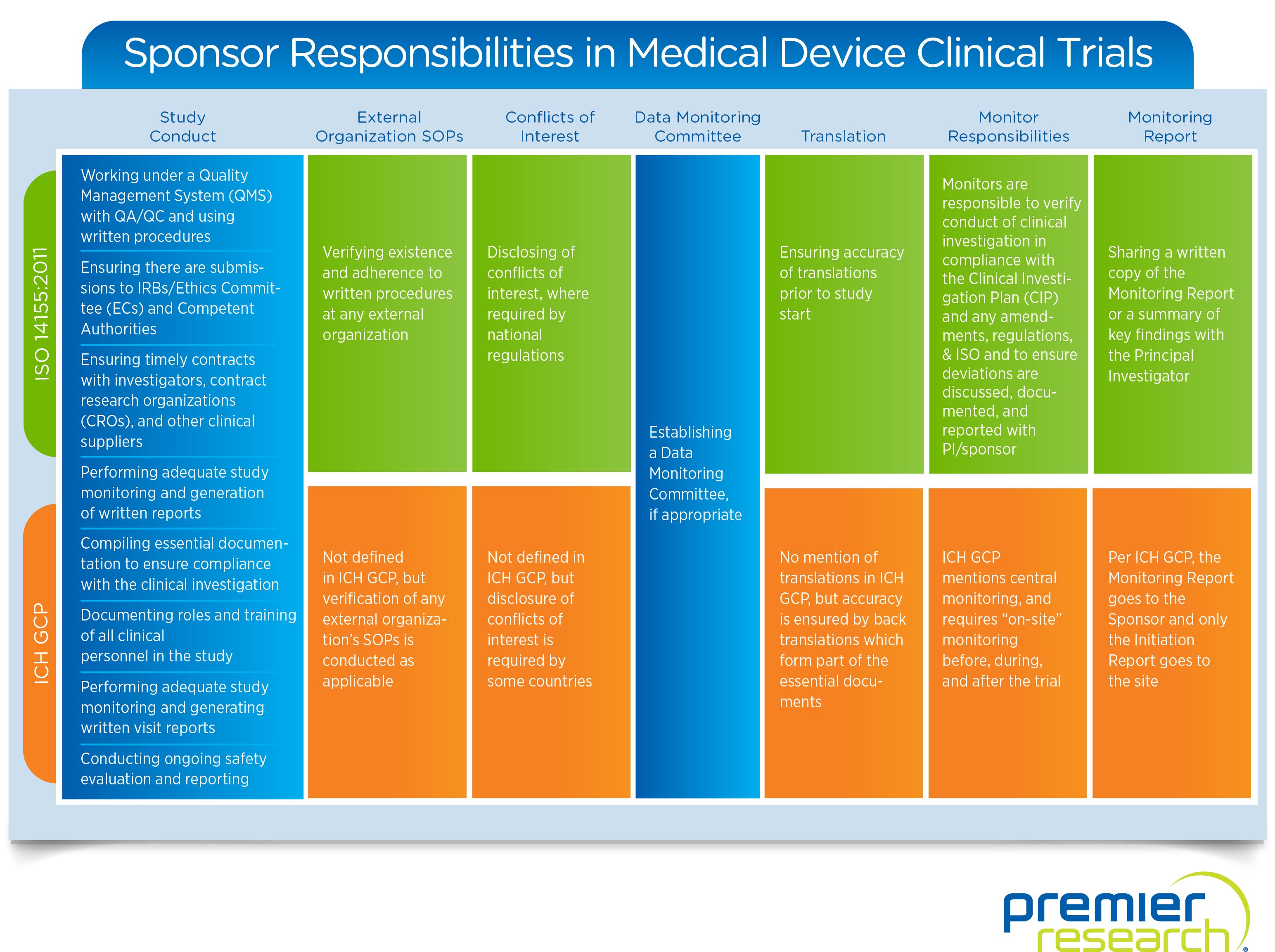
Roles of a Sponsor
The seven key jobs a sponsor takes on in a medical device trial are overseeing:
1. Study Conduct — Providing documentation, reviewing contracts, and checking regulatory compliance. The biggest role and the one that ties everything together.
2. External Organization SOPs — Ensuring consistency of and adherence to written procedures.
3. Conflicts of Interest — Identifying and disclosing any potential conflicts.
4. Data Monitoring — Creating independent data monitoring committees.
5. Translation — Ensuring initial accuracy or reviewing back translations.
6. Monitoring — Verifying adherence to study conduct and applicable regulations.
7. Monitoring Reports — Providing written summaries or copies of relevant reports to the correct entities.
What About Funding?
You may be wondering why “funding” is missing from this list. While often conflated, regulatory sponsors and financial sponsors aren’t the same by default. When used without qualification, “sponsor” usually specifically means “regulatory sponsor.”
ISO vs. ICH
While specific sponsor responsibilities outlined by ISO’s 14155:2011 and ICH’s GCP guidelines cover much of the same ground, they do have some key differences. For a more in-depth look at how these compare, take a look at this infographic: