It would be hard to overstate the need for new therapies that target unmet medical needs, especially in rare disease. After all, there are only about 400 approved “orphan drugs,” meaning that 95 percent of rare diseases lack a single approved treatment.
Now the good news: U.S. and European regulators, recognizing the size and severity of the need, offer provisions to mitigate the enormous expense and extended timelines for reviewing and approving these drugs. The U.S. Food and Drug Administration and the European Medicines Agency have adopted similar strategies for expediting approvals and giving patients earlier access to life-changing, even life-saving, new drugs.
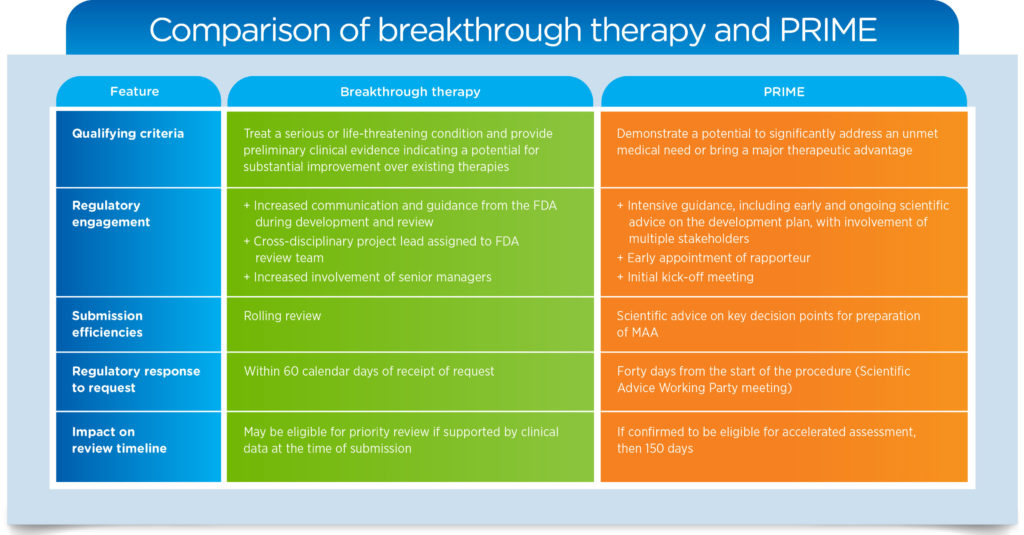
You can see the major elements of these provisions above — the FDA’s breakthrough therapy designation and the EMA’s PRIME (PRIority MEdicines) initiative — and how qualifying sponsors can best navigate these pathways. To learn more about this topic, download our white paper, Navigating Expedited Regulatory Pathways in the U.S. and Europe.
