The promise of gene therapy is to provide transformative treatments that meaningfully improve quality of life for patients, many of whom are currently living with debilitating diseases. To bring these treatments to market, sponsors are tasked with conducting clinical trials that generate robust evidence with appropriate safety oversight. In addition, gene therapy trials are faced with the myriad logistical hurdles of finding the right patients, pairing them with qualified sites, and ensuring that they understand and can fulfill the study protocol requirements.
In this first part of a three-part blog series on gene therapy development, we explore the emerging landscape of gene therapy and discuss high-level considerations for studies of these advanced therapeutics.
Current state of gene therapy
According to the National Institutes of Health, rare diseases are those that affect less than 200,000 patients in the U.S. Approximately 7,000 diseases meet this criterion and 80 percent of them have genetic origins. An estimated 95 percent of rare diseases currently have no treatment, underscoring the need for innovative treatments such as gene therapies.
Cellular and gene therapy development is a relatively new and rapidly evolving area of clinical trial activity, as evidenced by the FDA’s seven draft guidance documents in 2020. Yet, despite the large number of candidate therapies in development, few have made it to market and many hurdles remain.
Challenges in gene therapy
Hurdles in the development of gene therapy include:
- Manufacturing scalability. There remains a significant need and opportunity for finding efficiencies and optimizing manufacturing processes to deploy gene therapies cost-effectively and at scale.
- Complex regulatory pathways. The regulatory environment continues to evolve, and many jurisdictions lack an established regulatory framework for developing gene therapy products.
- Immunogenicity concerns. Immune responses directed against gene therapy products are multifactorial and often require long-term follow-up.
- Dosing determination. Determining a safe, effective dose can be challenging as preclinical data may not translate to humans and high dosing can lead to serious health risks.
Big picture considerations for gene therapy clinical trials
At a high level, when developing a gene therapy and designing a clinical study, sponsors should consider:
1. Type of vector
Two of the most common virus types used in the treatment of genetic disorders are lentivirus and adeno-associated virus (AAV). The type of vector used may impact both route of administration and study design.
Lentivirus is usually used when large capsids are required. Typically, lentiviral vectors are used for ex vivo gene-modified cell therapies, where cells are harvested from the patient, modified, and then returned to the patient. Lentiviruses integrate their payload into the host chromosome, so therapies using lentiviral vectors require long-term follow-up of up to 15 years.
AAV vectors are usually administered in vivo, whether the route is intracranial, intraocular, intramuscular, or intravenous. Gene therapies using AAV vectors usually become an episome, a circular piece of DNA within the nucleus, and therefore require a follow-up of up to five years.
2. Location and number of sites
As patients, key opinion leaders (KOLs), and facilities with the necessary capabilities for running gene therapy studies are geographically dispersed, sponsors need to determine where to conduct their studies and how many sites to activate. The key is determining which principal investigators have conducted studies in the patient population of interest and whether they are affiliated with institutions that can run the study.
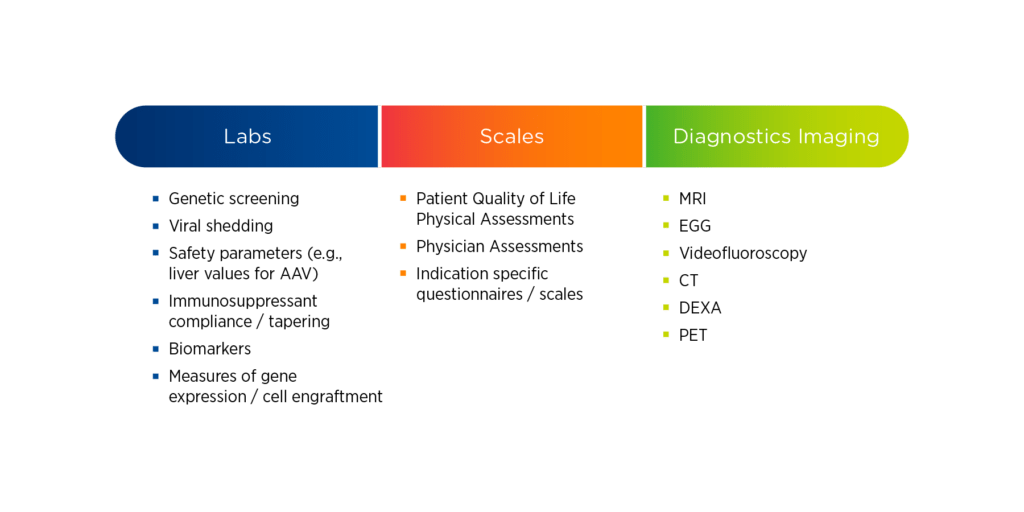
3. Types of assessments
Gene therapy studies are often resource-intensive, requiring sites to perform a variety of evaluations. These evaluations may include both simple and complex laboratory tests, scales and assessments, and specialty imaging. For each of these evaluations, it is essential to understand who is qualified to perform it, how frequently it needs to be performed, and where it can be performed.
Laboratory assessments can be complicated for fragile pediatric populations, therefore specialized vendors might be needed. For scales and assessments, sponsors should consider whether raters require special qualifications, how to handle inter-rater reliability, and whether these evaluations can be performed through home health or mHealth services. For specialty imaging requirements, specific calibrations, protocols, and validations may be necessary; which may limit the locations where imaging can be performed.
Figure 1. Common assessments in gene therapy studies
In part two of this blog series, we explore key considerations for the design of gene therapy trials.
At Premier, we have conducted more than 60 cell and gene therapy studies in the past five years. Our significant experience in viral vectors is combined with deep therapeutic expertise in oncology, rare disease and pediatric research. Click here to learn more about how Premier can help move gene therapy research forward.
