Designing and conducting a gene therapy trial is a complex undertaking. Understanding, planning for, and overcoming the myriad challenges of operationalizing these studies will help you bring safe, breakthrough treatments to patients with unmet medical needs.
In this blog post, we introduce a case study as a framework for exploring critical study design considerations of gene therapy trials and offering strategies for addressing those hurdles.
Background
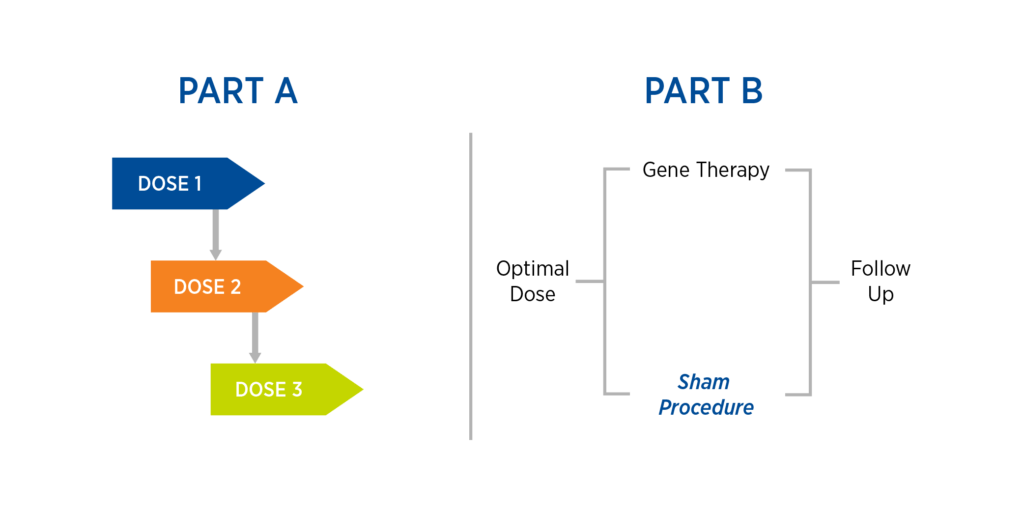
This case study is based on Premier Research’s work operationalizing a rescue Phase 1/2 gene therapy trial involving localized administration of gene therapy using specialized equipment. The study comprised two parts, the second of which includes a sham procedure (see Figure 1):
- Part A: A dose-escalation study consisting of three cohorts and dose levels, with safety reviews in between each dose
- Part B: Once an optimal dose is found, randomize study participants to either gene therapy or sham procedure with subsequent follow-up
Figure 1. Case study design
Notably, the protocol has undergone more than five amendments to date. The sham procedure in Part B was included at the request of the FDA. When the protocol was submitted in Western Europe, no major issues were identified. However, in one EU country, the Ethics Committee did not initially approve the use of a sham procedure. Premier worked with our in-country investigators, who all agreed to submit letters of support regarding the use of a sham procedure in Part B. One key factor highlighted in these letters was that, while the patient population was considered fragile, use of a sham procedure would not cause undue burden given the limited therapeutic options available. Another important element was that utilization of a sham procedure allowed for placebo control.
Critical considerations for gene therapy study planning
Below are some of the critical considerations addressed during the course of study conduct.
Feasibility. We performed a re-evaluation of the geographic areas where the study would be conducted and confirmed that both North American and Western Europe had a high incidence of the disease in question. We then re-established communications with these countries and sites to submit the appropriate documentation.
Site Selection. Given the randomized nature of Part B of the trial, it was determined that additional countries and sites would be needed in Western Europe. The site selection process involved identifying and bringing on sites that had scientific and clinical experience with — and access to — patients with the disease under investigation. In addition, the process included screening for previous experience not only with gene therapy but also with meeting country and local requirements from both a regulatory and protocol design standpoint. Site selection criteria also covered the surgical techniques and specialized equipment needed for gene therapy administration and the sham procedure, as well as the facilities and standard operating procedures necessary for receiving, storing, and preparing the gene therapy.
Enrollment. Although there is a high incidence of the disease in question in North America and Western Europe, it was expected that many study participants would come from outside the borders where the trial was being conducted. With the advent of the internet and the growing influence of advocacy groups, an increasing number of patients are taking their care into their own hands and seeking clinical trials for which they might be eligible. Consequently, cross-border enrollment was important to consider, and processes and procedures needed to be put in place to ensure smooth transitions for these patients.
Long-Term Follow-Up. Gene therapy trials require long-term follow-up, with follow-up periods ranging from five to 15 years, depending on the country and the type of gene therapy used. Given that gene therapies are often investigated in patients with serious conditions, you will need to consider that a percentage of patients will not survive the long-term follow-up period.
Logistics of Study Drug Administration. Due to the specialized surgical procedure required for gene therapy administration or the sham procedure, a custom piece would be created for each patient in the study. As the trial included sites in Western Europe, we needed to ensure that the necessary equipment components received CE marks. To maximize efficiency and patient centricity, we decided on a hub-and-spoke model for this trial, where specialized surgical centers serve as the hubs for study drug administration and referral centers act as spokes for recruitment, screening, and local follow-up.
Manufacturing. Gene therapy manufacturing is an expensive endeavor, and designing the manufacturing process to be a continuum throughout the course of a clinical program is critical. This includes having a backup plan and backup to that backup plan, as well as having a rolling stability program for the drug that has been manufactured.
Stay tuned for our third post looking at the operational challenges of gene therapy trials during COVID-19. Premier Research has conducted 60 gene and cell therapy trials in the past five years in multiple therapeutic areas, including oncology, hematology, neurology, and rare disease. Click here to schedule a consultation with our gene therapy experts.
