Featured Guests
Stacy Weil
Senior Vice President,
Clinical Data Operations, Strategic Business Optimization
Stacy Weil translates Premier Research’s strategy into actionable data initiatives that enable internal and external customers to achieve the highest level of performance and success. Prior to joining Premier Research, Ms. Weil was Vice President of Clinical Data Operations at PatientiP.
Read More
Nach Davé, RPh., MSc
Vice President, Development Strategy
Nach Davé provides strategic and commercial input for Premier Research’s business activities. He brings more than 20 years of experience in the pharmaceutical and contract research industries to the position. He previously served as the Vice President of Regulatory at Premier and has been in leadership positions at both CROs and sponsor companies.
Read More
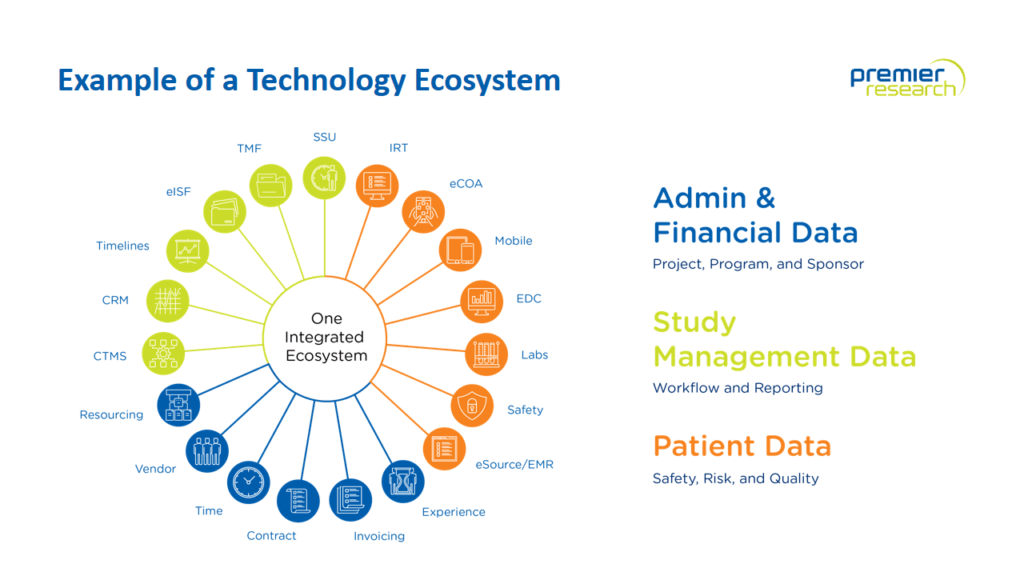
Click image to expand
Since early this year, many standard clinical trial processes have been significantly disrupted. Patients have been prevented from attending regular physician visits. Clinical research associates who traveled regularly to study sites to review and verify processes and data were unable to do so. Supply chain upheaval has forced schedule modifications and substitutions for products in short supply. Committees and boards accustomed to meeting in person were relegated to virtual gatherings, and clinical trial logistics had to be re-evaluated for time, cost, and quality expectations.
In these excerpts from their recent presentation, Premier Research’s Stacy Weil, Senior Vice President, Clinical Data Operations, Strategic Business Optimization, and Nach Dave, RPh., MSc, Vice President, Development Strategy, discuss the need to change the way patients participate in trials and how we collect and monitor data.
Schedule a consultation and coffee’s on us. Learn more and watch the full recording here.