Product Development & Regulatory Consulting
Offering decades of strategic development experience, global compliance, and therapeutic experience to ensure your promising therapy isn’t delayed by risk or limited resources.
WEBINAR
Achieving commercial success
Preparing your market application and planning the lifecycle management of your development program are critical to moving your product through early-phase trials and achieving commercial success.
PREMIER PERSPECTIVE
Product development checklist
A comprehensive plan and the right regulatory and therapeutic expertise can significantly accelerate the development timeline and increase the likelihood of marketing success, especially for small biotech and specialty pharma companies working with limited time and resources.
Taking your product to the next level
Our consultants work with you to design and implement comprehensive plans to enable successful product development from early discovery through IND and post-approval lifecycle management, supporting the regulatory pathway and tactical implementation.
Related Capabilities
Adding value at every step in the process, we provide expertise in global regulatory consulting, clinical R&D, and technology tools to ensure your trial’s data integrity, process efficiency, and timely analytics and reporting.
Global Regulatory Consulting

Clinical Research & Development
Premier Ecosystem
Therapeutic Focus
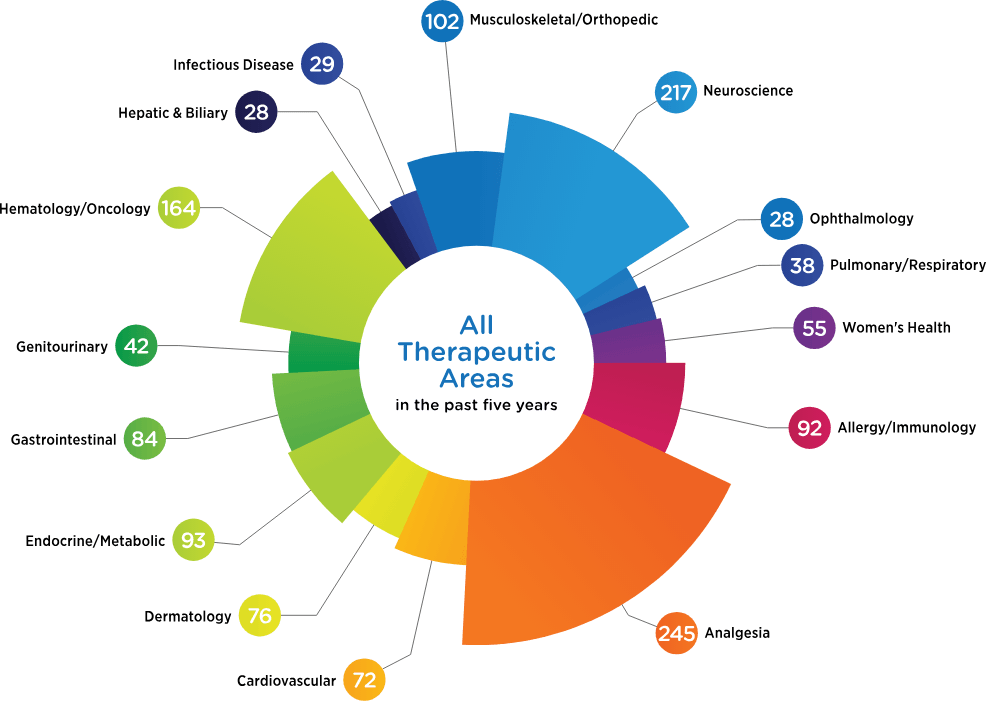
Therapeutic Focus
There’s no substitute for experience. And we have a lot of it. See what we’ve been busy doing for the past five years.
Check out our resource center
Our experts have developed an extensive library of white papers, case studies, blogposts, and other informative resources.
CASE STUDIES
WHITE PAPERS
WEBINARS
VIDEOS
PODCASTS
Connect with us
Ready to get started? So are we. Drop us a line to learn more about how we can help.




